Kasba Gangrape: Court sends all 3 accused to 7-day police custody
.gif)
.gif)
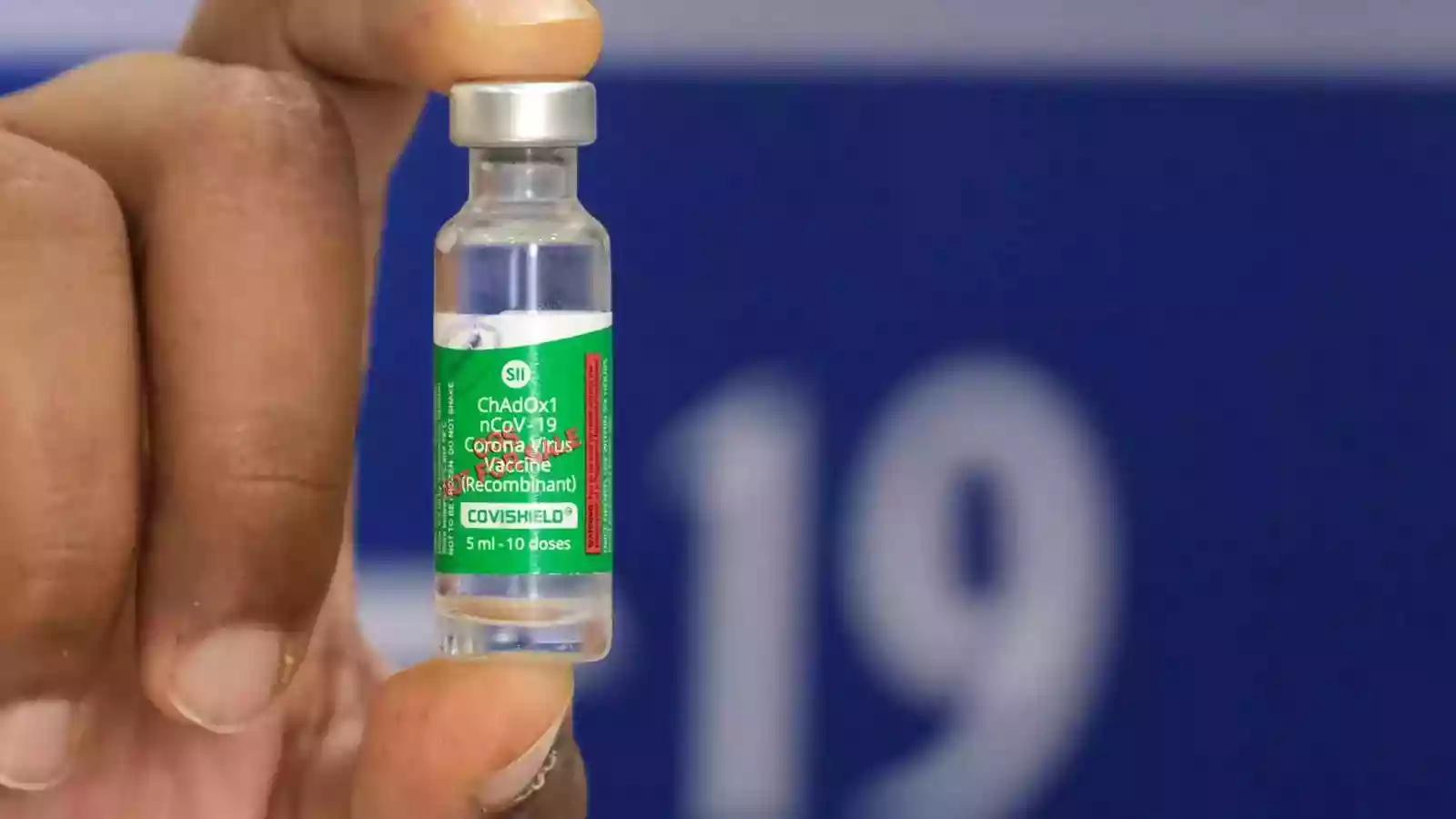
A significant revelation has surfaced regarding the COVID vaccine and its rare side effects, raising concerns among health experts. AstraZeneca, a British-Swedish pharmaceutical giant, has acknowledged in court documents that its COVID vaccine, Covishield, may lead to a 'rare side effect' associated with blood clots.
Covishield, developed by AstraZeneca and produced by the Serum Institute of India, was among the widely administered COVID vaccines in India. However, AstraZeneca is currently facing a class action lawsuit in the UK over allegations linking its vaccine to fatalities, with 51 cases filed against the company.
In a court filing, AstraZeneca conceded that Covishield can, "in very rare cases, cause TTS," referring to Thrombosis with Thrombocytopenia Syndrome. According to Dr. Viswesvaran B, Consultant Interventional Pulmonology and Sleep Medicine at Yashoda Hospitals Hyderabad, TTS is an extreme immunological reaction that may occur after COVID-19 immunization, potentially leading to blood clotting issues and hemorrhages.
Speaking on the symptoms, the expert says, "Persistent and progressively worsening headache in addition to focal neurological symptoms like visual disturbances are described as early red flags for suspecting VITT in patients."
Symptoms also include are like severe headache, stroke, abdominal pain, swelling of legs and severe breathlessness. TTS patients requires interdisciplinary collaboration between cardiologists, haematologists, neurologists, and other allied health specialists for treatment.